Equine Neuromuscular Diagnostic Laboratory
Valberg Neuromuscular Diagnostic Laboratory
Dedicated to providing the most accurate diagnosis and optimal treatment of muscle disorders in horses
Valberg Neuromuscular Diagnostic Laboratory
Dedicated to providing the most accurate diagnosis and optimal treatment of muscle disorders in horses
Valberg Neuromuscular Diagnostic Laboratory
Dedicated to providing the most accurate diagnosis and optimal treatment of muscle disorders in horses
Welcome to the Valberg Neuromuscular Diagnostic Laboratory (NMDL). The NMDL is dedicated to providing the most accurate diagnosis and optimal treatment of muscle disorders in horses.
Welcome to the Valberg Neuromuscular Diagnostic Laboratory (NMDL). The NMDL is dedicated to providing the most accurate diagnosis and optimal treatment of muscle disorders in horses.
Fill out the Muscle Biopsy Submission Form. You will receive a PDF of the completed form to print and submit with the biopsy.
Fees: $300 per biopsy sample submitted.
INFORMATION ON SUBMITTING A SAMPLE
What muscle should I biopsy?
- For exertional rhabdomyolysis, polysaccharide storage myopathy, myofibrillar myopathy: semimem/tendinosus, middle gluteal muscle
- For generalized atrophy: sacrocaudalis dorsalis medialis muscle
- For focal muscle atrophy: the atrophied muscle
How do I handle the muscle sample?
- Beginning July 1, 2022, we will only accept formalin-fixed samples. We have perfected IHC stains for formalin-fixed tissue and can diagnose equine muscle diseases using these stains on a smaller sample
- Obtain a 1/2-inch cube of semimembranosus or sacrocaudalis (if atrophy)
- Leave biopsy in air while you suture
- Optional, cut the biopsy in half across with width of the muscle (not lengthwise) and place in the cassette so the two halves can be sectioned simultaneously.
- Place in at least 130 ml (4.5 oz) 10% neutral buffered formalin 3-5 min after you take the biopsy
When should we schedule a biopsy?
Biopsies can now be scheduled anytime. IHC stains work best on samples that have not been in formalin for more than 5 days.
The Process
1. Submit form online
2. Receive email with instructions and paperwork to accompany samples
3. Send samples to MSU Integrated histopathology lab for slide preparation (not the MSU Veterinary Diagnostic Laboratory as was done prior to January 2023)
4. Slides collected from histopathology lab and read by Dr. Valberg
5. Report of results for paid submissions sent by Dr. Valberg to submitting veterinarian
Kentucky Equine Research provides administrative services to the Valberg NMDL and does not receive specimens nor is it party to the results. Please do not send any samples to Kentucky Equine Research.
Percutaneous Needle Biopsy (Middle Gluteal Muscle)
Please download the Percutaneous Needle Biopsy Technique PDF for reference.
Semimembranosus/Semitendinosus
The best site for a semimembranosus biopsy is midway between the tuber ischii and the origin of the Achilles tendon at about the level of the vulvar lips. Avoid tendinous insertions. This site hides scarring under tail hairs and is easily treated if dehiscence occurs.
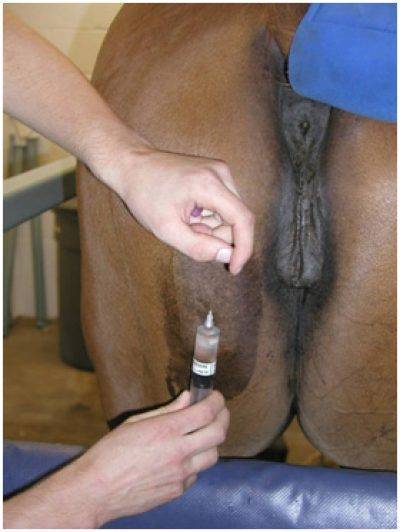
Sacrocaudalis
The best site for a sacrocaudalis biopsy is 1/2 inch above the origin of the tail and approximately 1/4 inch off midline. Skin retractors are highly recommended for this site.
Procedure
- Lidocaine is injected under the skin but not into the muscle belly. This area has many nerves so 5-7 ml of lidocaine may be necessary.
- A 2-inch incision is made through the skin and subcutaneous fat and fascia. The skin is not flexible in this area so a larger skin incision is needed.
- Use skin retractors for visualization.
- Parallel longitudinal incisions are made in the muscle 1/4 inch apart.
- The cranial aspect of the muscle is grasped with forceps and the muscle is dissected out 1/4 inch deep and 1/2 inch long. Don’t pull this muscle out via forceps as that squishing creates atrophy.
- This area can have a lot of subcutaneous fat. Make sure the sample is deep enough to obtain muscle tissue.
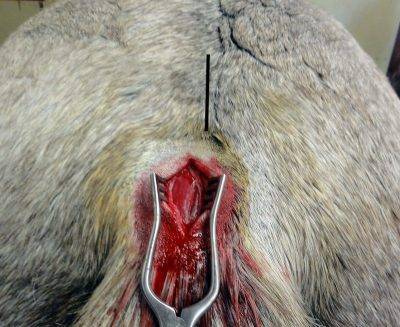
- Do not squeeze or squish as that can damage cells. Do not ship muscle in red top tubes.
- Place a ½” cube of fresh muscle in at least 130 ml (4.5 oz) of 10% buffered formalin in a hard, watertight container. Larger samples than this won’t fix well so trim them.
- Label each container with the owner’s name, horse’s name, and name of the muscle it contains (one muscle per container).
- Wrap the top with parafilm and place it in a biohazard Ziploc bag containing absorbent material such as paper towel.
- Fill out the submission form, submit and print out the PDF that you receive. Send in a separate Ziploc bag with the sample.
- Sample submissions do not have to be shipped overnight. For us to track the biopsies closely upon arrival at MSU, please DO NOT arrange for a Saturday or Sunday delivery. Samples can stay in formalin over the weekend and be shipped on Monday.
- Ship to:
- Michigan State University
Departments of Physiology & Pathology
Investigative HistoPathology Lab
567 Wilson Road – Rm 1104
East Lansing, MI 48824
- Michigan State University
NOTE: A permit is required when shipping originates outside the United States. If shipping samples from outside the USA, six unstained parrafin-embeded slides sent in a plastic slide container can be sent to the Valberg NMDL and will be stained here at the same cost as a muscle sample submission.
For shipments from Canada: Please email [email protected] to receive an import permit. We strongly recommend using a major carrier such as FedEx to ensure the samples clear customs.
NOTE: You must include with the formalin-fixed samples:
- The IMPORT PERMIT
- An ORIGINAL signed document from the producer/manufacturer that CLEARLY corresponds to the shipment by means of an invoice number or shipping marks or lot number or other identification method. This document must confirm that:
- The exported materials are formalin fixed muscle samples derived from equine species that:
- Originated in Canada,
- Were not known to be or suspected of having been exposed to or infected with any specific animal pathogen.
- The exported materials were not exposed to or commingled with any other animal derived material.
- The exported materials are formalin fixed muscle samples derived from equine species that:
- The submitter is responsible for all fees associated with the submission.
- Turnaround time for results is 10-14 days
- The submitter is responsible for adherence to sample-shipping regulations.
- The Valberg Neuromuscular Diagnostic Laboratory no longer offers a shipping discount code for sample submissions. We apologize for any inconvenience.
- All samples and specimens submitted become the property of Valberg Neuromuscular Diagnostic Laboratory and will not be returned.
- Samples, specimens, and related test and diagnostic results may be used for teaching and research purposes.
For any questions or concerns regarding submission, please email [email protected].
Percutaneous Needle Biopsy (Middle Gluteal Muscle)
Please download the Percutaneous Needle Biopsy Technique PDF for reference.
Semimembranosus/Semitendinosus
The best site for a semimembranosus biopsy is midway between the tuber ischii and the origin of the Achilles tendon at about the level of the vulvar lips. Avoid tendinous insertions. This site hides scarring under tail hairs and is easily treated if dehiscence occurs.

Sacrocaudalis
The best site for a sacrocaudalis biopsy is 1/2 inch above the origin of the tail and approximately 1/4 inch off midline. Skin retractors are highly recommended for this site.
Procedure
- Lidocaine is injected under the skin but not into the muscle belly. This area has many nerves so 5-7 ml of lidocaine may be necessary.
- A 2-inch incision is made through the skin and subcutaneous fat and fascia. The skin is not flexible in this area so a larger skin incision is needed.
- Use skin retractors for visualization.
- Parallel longitudinal incisions are made in the muscle 1/4 inch apart.
- The cranial aspect of the muscle is grasped with forceps and the muscle is dissected out 1/4 inch deep and 1/2 inch long. Don’t pull this muscle out via forceps as that squishing creates atrophy.
- This area can have a lot of subcutaneous fat. Make sure the sample is deep enough to obtain muscle tissue.

Fill out the Muscle Biopsy Submission Form. You will receive a PDF of the completed form to print and submit with the biopsy.
Fees: $475 per biopsy (each muscle sample) submitted.
Biopsy submissions may take up to 10 business days to be processed, once received. Results will be sent by email.
INFORMATION ON SUBMITTING A SAMPLE
What muscle should I biopsy?
- For exertional rhabdomyolysis, polysaccharide storage myopathy, myofibrillar myopathy: semimem/tendinosus, middle gluteal muscle
- For generalized atrophy: sacrocaudalis dorsalis medialis muscle
- For focal muscle atrophy: the atrophied muscle
How do I handle the muscle sample?
- We will only accept formalin-fixed samples. We have perfected IHC stains for formalin-fixed tissue and can diagnose equine muscle diseases using these stains on a smaller sample
- Obtain a 1/2-inch cube of semimembranosus or sacrocaudalis (if atrophy)
- Leave biopsy in air while you suture
- Optional, cut the biopsy in half across with width of the muscle (not lengthwise) and place in the cassette so the two halves can be sectioned simultaneously.
- Place in at least 130 ml (4.5 oz) 10% neutral buffered formalin 3-5 min after you take the biopsy
When should we schedule a biopsy?
Biopsies can now be scheduled anytime. IHC stains work best on samples that have not been in formalin for more than 5 days.
Percutaneous Needle Biopsy (Middle Gluteal Muscle)
Please download the Percutaneous Needle Biopsy Technique PDF for reference.
Surgical Biopsy
Semimembranosus/Semitendinosus
The best site for a semimembranosus biopsy is midway between the tuber ischii and the origin of the Achilles tendon at about the level of the vulvar lips. Avoid tendinous insertions. This site hides scarring under tail hairs and is easily treated if dehiscence occurs.

Sacrocaudalis
The best site for a sacrocaudalis biopsy is 1/2 inch above the origin of the tail and approximately 1/4 inch off midline. Skin retractors are highly recommended for this site.
Surgical Procedure
- Lidocaine is injected under the skin but not into the muscle belly. This area has many nerves so 5-7 ml of lidocaine may be necessary.
- A 2-inch incision is made through the skin and subcutaneous fat and fascia. The skin is not flexible in this area so a larger skin incision is needed.
- Use skin retractors for visualization.
- Parallel longitudinal incisions are made in the muscle 1/4 inch apart.
- The cranial aspect of the muscle is grasped with forceps and the muscle is dissected out 1/4 inch deep and 1/2 inch long. Don’t pull this muscle out via forceps as that squishing creates atrophy.
- This area can have a lot of subcutaneous fat. Make sure the sample is deep enough to obtain muscle tissue.

- Do not squeeze or squish as that can damage cells. Do not ship muscle in red top tubes.
- Place a ½” cube of fresh muscle or a tissue cassette containing specimen collected from needle biopsy in at least 130 ml (4.5 oz) of 10% buffered formalin in a hard, watertight container. Larger samples than this won’t fix well so trim them.
- Label each container with the owner’s name, horse’s name, and name of the muscle it contains (one muscle per container).
- Wrap the top with parafilm and place it in a biohazard Ziploc bag containing absorbent material such as paper towel.
- Fill out the submission form online, submit and print out the PDF that you receive. Send in a separate Ziploc bag with the sample.
- Sample submissions do not have to be shipped overnight. For us to track the biopsies closely upon arrival, please DO NOT arrange for a Saturday or Sunday delivery. Samples can stay in formalin over the weekend and be shipped on Monday.
- Ship to:
- Melbourne Veterinary School
Histopathology Laboratory
University of Melbourne, Australia
250 Princes Hwy
Werribee, VIC 3030Tel: +61 3 9731 2274
- Melbourne Veterinary School
- The submitter is responsible for all fees associated with the submission.
- Turnaround time for results is 10-14 days
- The submitter is responsible for adherence to sample-shipping regulations.
- All samples and specimens submitted become the property of Valberg Neuromuscular Diagnostic Laboratory and will not be returned.
- Samples, specimens, and related test and diagnostic results may be used for teaching and research purposes.
For any questions or concerns regarding submission, please email [email protected].
Fill out the Muscle Biopsy Submission Form. You will receive a PDF of the completed form to print and submit with the biopsy.
Fees: $475 per biopsy (each muscle sample) submitted.
Biopsy submissions may take up to 10 business days to be processed, once received. Results will be sent by email.
INFORMATION ON SUBMITTING A SAMPLE
What muscle should I biopsy?
- For exertional rhabdomyolysis, polysaccharide storage myopathy, myofibrillar myopathy: semimem/tendinosus, middle gluteal muscle
- For generalized atrophy: sacrocaudalis dorsalis medialis muscle
- For focal muscle atrophy: the atrophied muscle
How do I handle the muscle sample?
- We will only accept formalin-fixed samples. We have perfected IHC stains for formalin-fixed tissue and can diagnose equine muscle diseases using these stains on a smaller sample
- Obtain a 1/2-inch cube of semimembranosus or sacrocaudalis (if atrophy)
- Leave biopsy in air while you suture
- Optional, cut the biopsy in half across with width of the muscle (not lengthwise) and place in the cassette so the two halves can be sectioned simultaneously.
- Place in at least 130 ml (4.5 oz) 10% neutral buffered formalin 3-5 min after you take the biopsy
When should we schedule a biopsy?
Biopsies can now be scheduled anytime. IHC stains work best on samples that have not been in formalin for more than 5 days.
Percutaneous Needle Biopsy (Middle Gluteal Muscle)
Please download the Percutaneous Needle Biopsy Technique PDF for reference.
Surgical Biopsy
Semimembranosus/Semitendinosus
The best site for a semimembranosus biopsy is midway between the tuber ischii and the origin of the Achilles tendon at about the level of the vulvar lips. Avoid tendinous insertions. This site hides scarring under tail hairs and is easily treated if dehiscence occurs.

Sacrocaudalis
The best site for a sacrocaudalis biopsy is 1/2 inch above the origin of the tail and approximately 1/4 inch off midline. Skin retractors are highly recommended for this site.
Surgical Procedure
- Lidocaine is injected under the skin but not into the muscle belly. This area has many nerves so 5-7 ml of lidocaine may be necessary.
- A 2-inch incision is made through the skin and subcutaneous fat and fascia. The skin is not flexible in this area so a larger skin incision is needed.
- Use skin retractors for visualization.
- Parallel longitudinal incisions are made in the muscle 1/4 inch apart.
- The cranial aspect of the muscle is grasped with forceps and the muscle is dissected out 1/4 inch deep and 1/2 inch long. Don’t pull this muscle out via forceps as that squishing creates atrophy.
- This area can have a lot of subcutaneous fat. Make sure the sample is deep enough to obtain muscle tissue.

- Do not squeeze or squish as that can damage cells. Do not ship muscle in red top tubes.
- Place a ½” cube of fresh muscle or a tissue cassette containing specimen collected from needle biopsy in at least 130 ml (4.5 oz) of 10% buffered formalin in a hard, watertight container. Larger samples than this won’t fix well so trim them.
- Label each container with the owner’s name, horse’s name, and name of the muscle it contains (one muscle per container).
- Wrap the top with parafilm and place it in a biohazard Ziploc bag containing absorbent material such as paper towel.
- Fill out the submission form online, submit and print out the PDF that you receive. Send in a separate Ziploc bag with the sample.
- Sample submissions do not have to be shipped overnight. For us to track the biopsies closely upon arrival, please DO NOT arrange for a Saturday or Sunday delivery. Samples can stay in formalin over the weekend and be shipped on Monday.
- Ship to:
- Melbourne Veterinary School
Histopathology Laboratory
University of Melbourne, Australia
250 Princes Hwy
Werribee, VIC 3030
- Melbourne Veterinary School
Tel: +61 3 9731 2274
Muscle biopsy samples from New Zealand should be able to meet the conditions for shipping to Australia without an import permit.
Follow the steps below.
Please visit the BICON website.
Under “Search for Import Item” search for either of the following.
- Preserved and fixed human and animal specimens
- Select the option for “Other animal and invertebrate specimens”
- Select ‘Yes’ if the specimens have been preserved by one of the listed methods. This will take you to the importing conditions and outline what the requirements are to import the goods.
- Animal fluids and tissues (excluding viable reproductive materials)
- Select the option for “Animal fluids and tissues from New Zealand”
- Select “Animal fluids and tissues (excluding avian, porcine, foetal bovine sera and reproductive material)”
- This will take you to the importing conditions and outline what the requirements are to import the goods.
When selecting the options, ensure that the goods meet the description to ensure you’re provided with the correct BICON case/importing conditions.
A permit is not required for either pathway if you are able to meet the import conditions listed for each case and supply the relevant documentation. Please check you can comply with import conditions and documentation before taking the biopsy.
Note: We recommend listing the consignment number on the import documentation.
Email [email protected] for further assistance submitting samples from outside of Australia.
- The submitter is responsible for all fees associated with the submission.
- Turnaround time for results is 10-14 days
- The submitter is responsible for adherence to sample-shipping regulations.
- All samples and specimens submitted become the property of Valberg Neuromuscular Diagnostic Laboratory and will not be returned.
- Samples, specimens, and related test and diagnostic results may be used for teaching and research purposes.
For any questions or concerns regarding submission, please email [email protected].
Stephanie Valberg, D.V.M., Ph.D., Dipl. ACVIM, ACVSMR
Dr. Stephanie Valberg is an international leader in diagnosing and treating equine neuromuscular disorders. The overarching goal of Valberg’s research and clinical work is to define the basis for neuromuscular disorders in horses, develop accurate, minimally invasive diagnostic tests, and produce optimal methods for preventing or managing performance limiting diseases.
Note: We do not perform genetic testing. Please submit to the Veterinary Genetics Lab at UC Davis.
Information on this site is intended for information purposes for veterinarians to guide in the diagnostic workup of muscle diseases. Owner-diagnosis and owner-treatment of horses is not recommended and may indeed be dangerous. Owners must consult their veterinarian before implementing any of the treatment recommendations described.
About the Valberg Neuromuscular Diagnostic Laboratory
The NMDL is dedicated to providing the most accurate diagnosis and optimal treatment of muscle disorders in horses.